[the_ad id=”476″]
Concept of Evaporation
Evaporation is the change of a liquid into a gas at the surface. It occurs at any temperature but occurs more rapidly at higher temperatures because heat imparts more kinetic energy to the molecules and they escape from the surface faster. Evaporation results in the general loss in temperature,known as the Cooling effect of evaporation.
Increased gas pressure on the surface of the liquid reduces the rate of evaporation because more collisions occur between the evaporating liquid molecules and the gas molecules, resulting in some of the evaporated liquid molecules bouncing back into liquid.
Some factors increase the rate of evaporation namely:
- Increase in temperature: If we observe the wet clothes, we will notice that on a warm day, they dry faster than on a cold day. This is because more particles of the water have enough energy to escape.
- Increase in surface area: Water in a puddle on the road will dry more quickly than water in a cup. This is because more water molecules in the puddle are close to the surface and escape in bigger number than in the cup.
- Air blowing across the surface: When a day is windy, the clothes dry faster than when it is quiet or you can dry your wet hand by getting them closer to the fan. This is because the moving air carries escaping water molecules away from the liquid, preventing them from bouncing back into the liquid as result of air pressure over the liquid.
- Reduction of humidity: when air contains more water vapour, it is said to be very humid. Wet clothes will take longer to dry in very humid air because molecules in the water vapour return to the liquid almost at the same rate as water molecules escape from it.
During the evaporation process, the molecules that escape from the liquid are the most energetic ones. The average kinetic energy of those that remain is therefore reduced, and the temperature reduces. This is cooling effect of evaporation.
Evaporation results in a general cooling of the liquid. This cooling is brought about by the lowering of the overall kinetic energy of the liquid as the more energetic molecules escape from the surface.
If energy is supplied to the liquid molecules (for example by heating), the rate of evaporation increases. If this energy comes from the surroundings, then the surroundings lose energy and experience a drop in temperature. The cooling effect of evaporation can be demonstrated by the following experiment:
Experiment 1.4: Cooling effect of evaporation.
Aim: To demonstrate the cooling effect of evaporation.
Requirements: a small beaker, ether, wooden block, water, glass tube
Procedure:
Place a small beaker, about one third full of ether, on a flat piece of wood which has a layer of water on it. Place a glass tube obliquely into the beaker to allow air in the beaker.
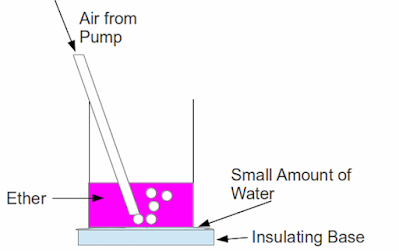
Observation:
Air is bubbled through the ether and effectively increases the surface area of the liquid and allows a lot more evaporation to take place. As the evaporation continues, the water between the beaker and wooden block will gradually start to freeze and the beaker will stick to the wooden block. Water droplets will also form on the outside of the beaker.
Explanation:
The energy required for this increased rate of evaporation is drawn from the beaker, the water and the wooden block
Water droplets form on the outside of the beaker due to condensation of water vapour from the surrounding air coming into contact with the beaker.
The cooling effect of evaporation is utilised in refrigerators. A refrigerator basically transfers heat from the inside cabinet to the outside environment. The principle involves a liquid that is constantly evaporating and thereby drawing energy (or heat) from the inside of the cabinet. The transfer of the heat from the inside to the outside is quite rapid and the inside becomes very cool. Once outside the cabinet the heat is transferred to the surrounding environment through the thin tube containing the vapour and the vapour is condensed back into liquid. A pumping mechanism ensures the movement of the liquid vapour round a closed system of conducting pipes thereby keeping the same liquid flowing continually.
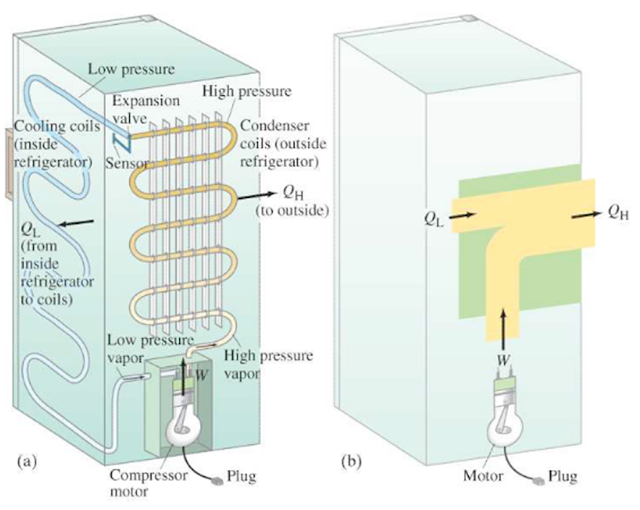
The figure above represents a typical refrigerator system. The electric compressor motor forces a gas at high pressure through a heat exchanger (condenser) on the rear outside wall of the refrigerator, where QH is given off, and the gas cools to become liquid. The liquid passes from a high-pressure region, via a valve, to low-pressure tubes on the inside walls of the refrigerator; the liquid evaporates at this lower pressure and thus absorbs heat (QL) from the inside of the refrigerator. The fluid returns to the compressor, where the cycle begins again
Other Cooling effects of Evaporation
Another every day application of cooling by evaporation is well observed in sweating: When animals sweat, fine drops of moisture are released either through the skin or through pores on the tongue. The passage of air over these drops results in evaporation which causes a cooling of the animal’s body in a similar way as described in the experiment above. Dogs, for example, remove excess heat from their bodies by panting.
Discover more from Centre for Elites
Subscribe to get the latest posts sent to your email.
